July 11, 2019 feature
Osteoblastic lysosome plays a central role in mineralization

Thamarasee Jeewandara
contributing writer
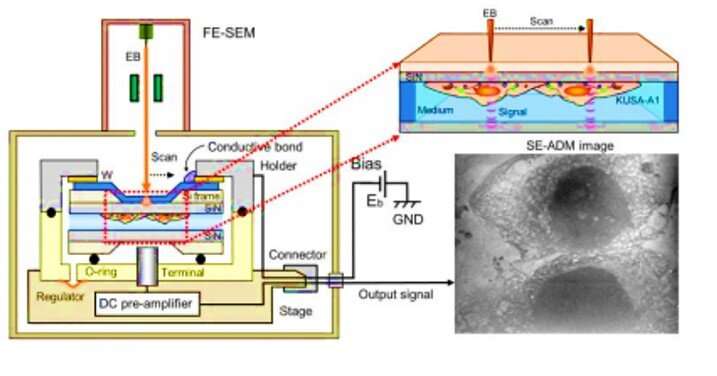
Mineralization is mediated by osteoblasts, which secrete mineral precursors through matrix vesicles (MVs) as a fundamental process in vertebrates. The vesicles are calcium and phosphate rich, containing organic materials such as acidic proteins. In a new study now published in Science Advances, Tomoaki Iwayama and colleagues at the departments of periodontology, biomedical research, oral science, biomaterials and oral anatomy development used scanning electron-assisted dielectric microscopy (SE-ADM) and super-resolution microscopy (SRM) to assess live osteoblasts during conditions of mineralization at nano-level resolution. They found the calcium-containing vesicles to be multi-vesicular bodies containing mineralizing nanovesicles or matrix vesicles (MVs). According to the observations, the MVs could be transported together with lysosomes and secreted by exocytosis. Iwayama et al. presented proof that the lysosomes could transport amorphous calcium phosphate in mineralizing osteoblast cells.
During the physiological process of bone mineralization, the deposition of calcium phosphate crystals occurs in the extracellular matrix as a in all vertebrates. In 1967, biologists and , individually visualized mineral-related particles in the extracellular space using electron microscopy (EM). Scientists later recognized these particles as mineralizing nano-vesicles or matrix vesicles (MVs). During the past 50 years of EM studies on MVs, biologists have grappled to understand the mechanism of MV formation and secretion, which .
Clarifying the mineralizing process of live cells with EM is challenging since sample preparation for EM requires steps on both chemical fixation and alcoholic dehydration. The steps can induce artefacts and even dissolve or remove unstable mineral precursors leaving an organic scaffold known as a "". While scientists had successfully used the process of EM using fixed and dehydrated tissue to view the structure of , to , they must employ cryo-EM processes to avoid dehydration and facilitate costly, extremely fast cooling with small specimens.
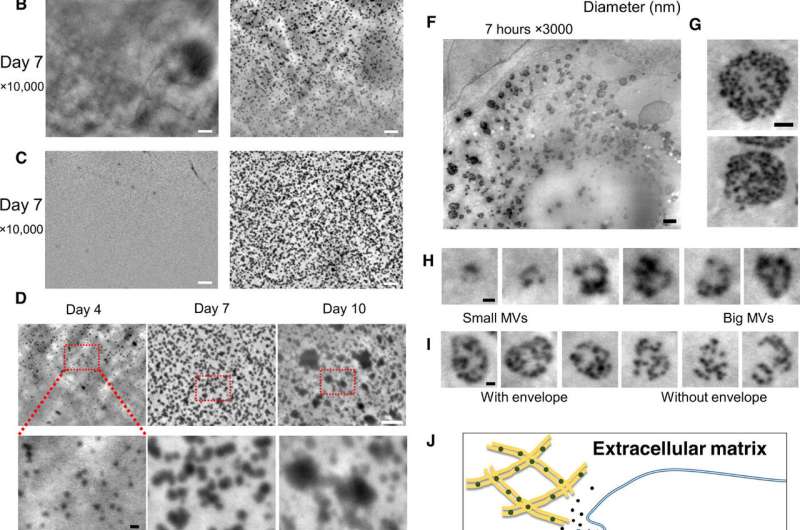
To overcome these limitations in the present work, Iwayama et al. used a new microscopic system known as scanning electron-assisted dielectric microscopy (SE-ADM). The method had previously and high-contrast imaging for mammalian cells in aqueous media without staining. The scientists used the same technique (high resolution SE-ADM) to explore the possibility of viewing MVs in intact osteoblasts to of MV trafficking. For the osteoblast cell line they used murine (mouse) , with high osteogenic capacity in vitro and in vivo. After cell culture under adequate conditions, Iwayama et al. observed the cells with SE-ADM to identify normal intracellular structures. The scientists observed MVs to align with collagen fibrils after 4 to 10 days of cell growth in osteogenic media and the secreted particle size increased due to fusion or particle growth, with their sizes consistent with previous reports to suggest .
Upon further examination with SE-ADM, they noted the involvement of the lysosomal pathway to transport and secrete intraluminal MVs in a similar process to exosomes. Interestingly, both exosomes and MVs are categorized as extracellular vesicles with similar sizes; they are both secreted by osteoblasts and have shared functions during .
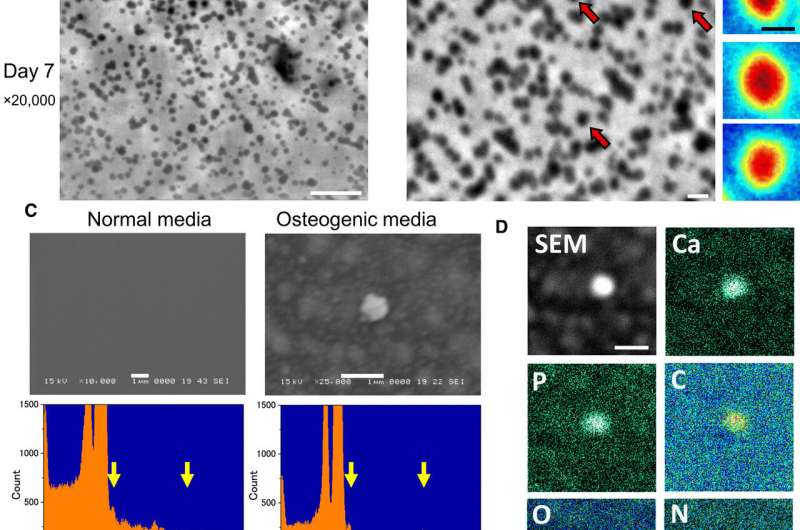
In the next step, Iwayama et al. examined if these particles were MVs containing calcium and/or phosphate. For this, they cultured the cells in osteogenic media for 7 days and observed them using SE-ADM to record very smooth structures without crystal facets. This suggested that the MVs did not crystallize but remained as also . When the scientists examined the MVs on a SiN (silicon mononitride) film, they observed sharp peaks corresponding to phosphorous, calcium, carbon and oxygen elements. They confirmed the findings using Raman spectroscopy to show the presence of calcium phosphate within MVs.
The scientists also investigated the effects of a medical condition encoded by the , wherein osteoblasts do not undergo mineralization in vitro. For this, they edited the genome of osteoblast cells using the CRISPR-Cas9 genome editing technology to generate Alpl knockout osteoblast clones. When Iwayama et al. examined the knockout clones using high-resolution SE-ADM, they did not observe MVs, which was further confirmed using spectrometric analysis due to the absence of phosphorous and calcium peaks.
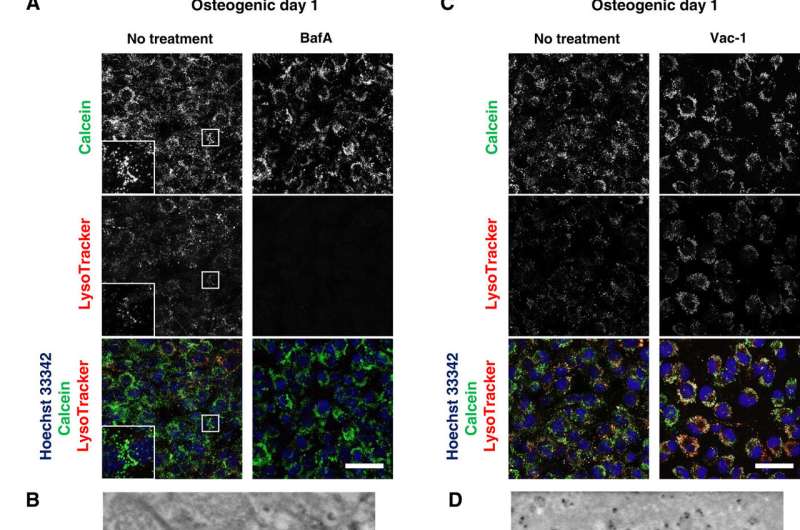
After directly observing the production and secretion of MVs using SE-ADM, the scientists further investigated the involvement of lysosomes in intracellular trafficking of MVs to observe live osteoblast mineralization. They cultured the cells in calcium-containing osteogenic media and stained them with to detect the intracellular components of interest. Iwayama et al. located the calcein-fulfilled vesicles matched with lysosomes to suggest the biogenesis of MVs within lysosomes after their fusion with calcein+ vesicles. The scientists followed the experiments with loss-of-function and functional inhibition studies to further deconstruct the pathways and examine intracellular mechanisms of action during live cell mineralization in vitro.
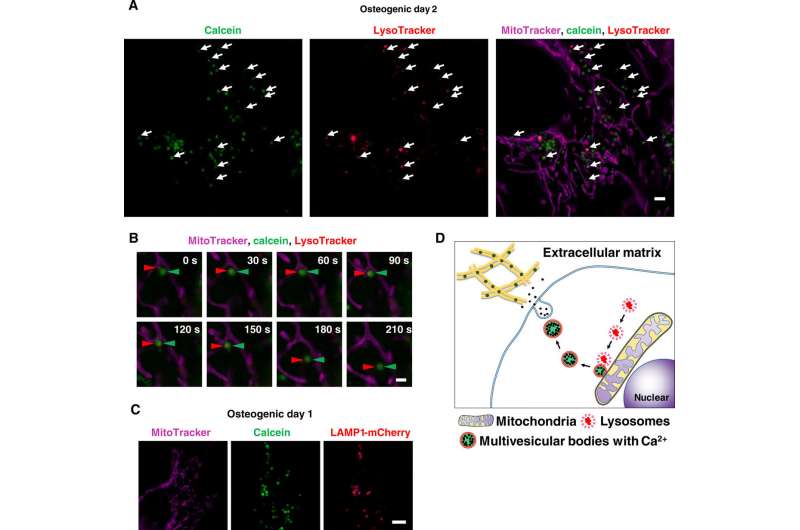
Scientists had previously reported the involvement of mitochondria during mineralization due to the presence of electron-dense calcium and phosphorous-rich granules in osteoblast mitochondria. This was observed with . Furthermore, reports also suggest the direct contact of lysosomes and mitochondria with functional significance. When Iwayama et al. stained cells with and observed the intracellular components under N-SIM structured illumination super-resolution microscopy (SRM). They observed the presence of most calcein-fulfilled vesicles next to mitochondria and matched with lysosomes. During SRM-time lapse imaging, the scientists further obtained views of intracellular transport of LysoTracker containing vesicles fused to static calcein vacuoles adjacent to mitochondria to validate their hypothesis.
In this way, together with observations of other SRM systems and additional cell lines, Tomoaki Iwayama and colleagues proposed a mineralization mechanism. Wherein lysosomes played a central role in intracellular MV biogenesis and trafficking within osteoblasts. It was reasonable to involve lysosomes for osteoblasts to transport amorphous calcium phosphate without crystallization during its transport in the cytosol. The scientists aim to conduct further experiments to understand the regulatory molecules for MVs and investigate if MVs and exosomes have similar constitutions and mechanism underlying their generation, secretion and function. The SE-ADM strategy used in the present work can be installed into existing scanning electron microscopy apparatus at a low cost. The work developed in the study will offer non-invasive, high-resolution imaging at the nanoscale applicable to all scientific fields.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Tomoaki Iwayama et al. Osteoblastic lysosome plays a central role in mineralization, Science Advances (2019).
N. Reznikov et al. A materials science vision of extracellular matrix mineralization, Nature Reviews Materials (2016).
Natalie Reznikov et al. Fractal-like hierarchical organization of bone begins at the nanoscale, Science (2018).
Irving M. Shapiro et al. Matrix vesicles: Are they anchored exosomes?, Bone (2015).
Journal information: Science Advances , Science , Bone
© 2019 Science X Network


















